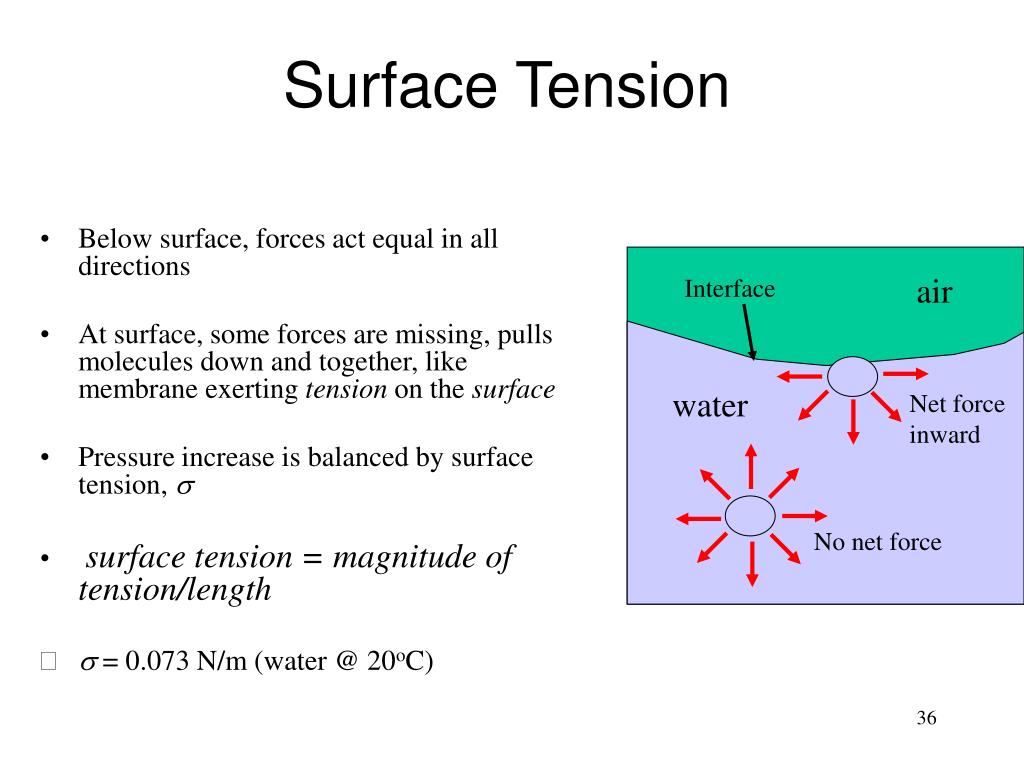
What Causes High Surface Tension Of Water . Water's high surface tension is due to the hydrogen bonding in. besides mercury, water has the highest surface tension for all liquids. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. Water consists of one oxygen atom flanked by two hydrogen. water has high surface tension, which can be explained by its polarity and hydrogen bonding. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Besides mercury, water has the highest surface tension for all liquids. surface tension, heat of vaporization, and vapor pressure. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). An example of such an. In comparison, organic liquids, such as benzene and alcohols , have lower.
from www.myxxgirl.com
water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water's high surface tension is due to the hydrogen bonding in. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. Besides mercury, water has the highest surface tension for all liquids. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension, heat of vaporization, and vapor pressure. In comparison, organic liquids, such as benzene and alcohols , have lower. An example of such an. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). Water consists of one oxygen atom flanked by two hydrogen.
Mechanical Properties Of Fluids Class Surface Tension Based On My XXX
What Causes High Surface Tension Of Water water has high surface tension, which can be explained by its polarity and hydrogen bonding. besides mercury, water has the highest surface tension for all liquids. Water consists of one oxygen atom flanked by two hydrogen. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension, heat of vaporization, and vapor pressure. An example of such an. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. Water's high surface tension is due to the hydrogen bonding in. Besides mercury, water has the highest surface tension for all liquids. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). In comparison, organic liquids, such as benzene and alcohols , have lower. water has high surface tension, which can be explained by its polarity and hydrogen bonding.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 What Causes High Surface Tension Of Water Besides mercury, water has the highest surface tension for all liquids. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). Water consists of one oxygen atom flanked by two hydrogen. An example of such an. water has a surface tension of 0.07275 joule. What Causes High Surface Tension Of Water.
From lessonlibnanometres.z13.web.core.windows.net
How To Explain Surface Tension What Causes High Surface Tension Of Water Water consists of one oxygen atom flanked by two hydrogen. water has high surface tension, which can be explained by its polarity and hydrogen bonding. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). besides mercury, water has the highest surface tension for all liquids. An example of such an.. What Causes High Surface Tension Of Water.
From www.youtube.com
Surface tension of water YouTube What Causes High Surface Tension Of Water surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). besides mercury, water has the highest surface tension for all liquids. Besides mercury, water has the highest surface tension for all liquids. Water consists of one oxygen atom flanked by two hydrogen. Water's high. What Causes High Surface Tension Of Water.
From studyschoolarborists.z1.web.core.windows.net
Tension And Surface Tension What Causes High Surface Tension Of Water surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). Besides mercury, water has the highest surface tension for all liquids. In comparison, organic liquids, such as benzene and alcohols , have lower. besides mercury, water has the highest surface tension for all liquids.. What Causes High Surface Tension Of Water.
From dxoxudzok.blob.core.windows.net
Surface Tension Of Water Pas at Debra Santiago blog What Causes High Surface Tension Of Water An example of such an. surface tension, heat of vaporization, and vapor pressure. Water's high surface tension is due to the hydrogen bonding in. Besides mercury, water has the highest surface tension for all liquids. In comparison, organic liquids, such as benzene and alcohols , have lower. besides mercury, water has the highest surface tension for all liquids.. What Causes High Surface Tension Of Water.
From www.youtube.com
The Meaning of Surface Tension and its Practical Applications YouTube What Causes High Surface Tension Of Water Water consists of one oxygen atom flanked by two hydrogen. In comparison, organic liquids, such as benzene and alcohols , have lower. water has high surface tension, which can be explained by its polarity and hydrogen bonding. An example of such an. Besides mercury, water has the highest surface tension for all liquids. surface tension of water can. What Causes High Surface Tension Of Water.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy What Causes High Surface Tension Of Water Water consists of one oxygen atom flanked by two hydrogen. besides mercury, water has the highest surface tension for all liquids. An example of such an. In comparison, organic liquids, such as benzene and alcohols , have lower. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on. What Causes High Surface Tension Of Water.
From www.studocu.com
Experiment 2 (chem) chemistry practical file Aim Determine the What Causes High Surface Tension Of Water water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Besides mercury, water has the highest surface tension for all liquids. An example of such an. surface tension, heat of vaporization, and vapor pressure. Water consists of one oxygen atom flanked by two hydrogen. besides mercury, water has the highest surface. What Causes High Surface Tension Of Water.
From www.biolinscientific.com
Why is surface tension important? What Causes High Surface Tension Of Water Water's high surface tension is due to the hydrogen bonding in. surface tension, heat of vaporization, and vapor pressure. An example of such an. In comparison, organic liquids, such as benzene and alcohols , have lower. water has high surface tension, which can be explained by its polarity and hydrogen bonding. water has a high surface tension. What Causes High Surface Tension Of Water.
From www.australianenvironmentaleducation.com.au
Water is found everywhere on earth, so why is it important? What Causes High Surface Tension Of Water water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. water has high surface tension, which can be explained by its polarity and hydrogen bonding. In comparison, organic liquids, such as benzene and alcohols , have lower. Water consists of one oxygen atom flanked by two hydrogen. surface tension. What Causes High Surface Tension Of Water.
From www.moleaer.com
How nanobubbles improve water infiltration by reducing surface tension What Causes High Surface Tension Of Water surface tension, heat of vaporization, and vapor pressure. Water consists of one oxygen atom flanked by two hydrogen. An example of such an. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Besides mercury, water has the highest surface tension for all liquids. Water's high surface tension is due to the. What Causes High Surface Tension Of Water.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii What Causes High Surface Tension Of Water surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). Water consists of one oxygen atom flanked by two hydrogen. water has high surface tension, which can be explained by its polarity and hydrogen bonding. besides mercury, water has the highest surface tension. What Causes High Surface Tension Of Water.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download What Causes High Surface Tension Of Water water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. In comparison, organic liquids, such as benzene and alcohols , have lower. surface tension, heat of vaporization, and vapor pressure. water has high surface tension, which can be explained by its polarity and hydrogen bonding. Besides mercury, water has. What Causes High Surface Tension Of Water.
From www.moleaer.com
How nanobubbles improve water infiltration by reducing surface tension What Causes High Surface Tension Of Water Water's high surface tension is due to the hydrogen bonding in. In comparison, organic liquids, such as benzene and alcohols , have lower. Besides mercury, water has the highest surface tension for all liquids. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. besides mercury, water has the highest. What Causes High Surface Tension Of Water.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance What Causes High Surface Tension Of Water water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). besides mercury, water has the highest surface tension for all liquids. surface tension, heat of vaporization, and vapor pressure. In comparison, organic liquids, such as benzene and alcohols , have lower. An example of such an. Water's high surface tension is. What Causes High Surface Tension Of Water.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts What Causes High Surface Tension Of Water water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Besides mercury, water has the highest surface tension for all liquids. In comparison, organic liquids, such as benzene and alcohols , have lower. An example of such an. Water's high surface tension is due to the hydrogen bonding in. besides mercury, water. What Causes High Surface Tension Of Water.
From www.myxxgirl.com
Mechanical Properties Of Fluids Class Surface Tension Based On My XXX What Causes High Surface Tension Of Water An example of such an. Water's high surface tension is due to the hydrogen bonding in. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). water has high surface tension, which can be explained by its polarity and hydrogen bonding. water has. What Causes High Surface Tension Of Water.
From ar.inspiredpencil.com
Surface Tension Water What Causes High Surface Tension Of Water water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water consists of one oxygen atom flanked by two hydrogen. An example of such an. besides mercury, water has the highest surface tension for all liquids. surface tension, heat of vaporization, and vapor pressure. Besides mercury, water has the highest surface. What Causes High Surface Tension Of Water.